27+ avogadro number calculator
Web number of particles Avogadro constant amount mol Example. 6022x1023 atoms 1 mole.

How To Find Oxidation Number Step By Step Explanation With Examples
And it contains 602214076 1023 number of particles whereas this number 602214076 1023 is called.

. Of different gases contain an equal number of molecules. Web Free Online Avogadros Number Calculator - A good calculator featured as part of our free online chemistry calculators each calculator can be used inline or full screen on. Web Free Online Avogadros Number Calculator - A good calculator featured as part of our free online chemistry calculators each calculator can be used inline or full screen on mobile.
Web One mole of oxygen atoms contain s 602214179 1023 oxygen atoms. Calculate the number of water molecules in 05 mol of. Number of water molecules 6022 10 23 05.
Web Use our online avogadros number calculator to find the no. Web One mole of H 2 O is 6022 x 10 23 molecules of H 2 O Avogadros number. Therefore we can use these conversion factors.
There are 6022x10 23 atoms in 1 mole of atoms. The measured value was. Web 1 mole of 12 x 16605 x 10-27 kg 602x1023 of 12 x 16605 x 10-27 kg 602x1023 x 12 x 16605 x 10-27 kg 0012 kg 12g Generally when working with.
Web This tool will calculate any parameter from the equation defined by Avogadros law which includes the V 1 gas volume n 1 amount of gas V 2 gas volume and n 2 amount of gas. Avogadros number eq6022times1023 eq is the number of particles in a mole. Its value is 60221023 units in each mole.
States that equal volumes. Calculate the volume of steam that can be produced from 50 cm 3 of. Until the 20th May of 2019 the Avogadro number was defined as the number of atoms of carbon in 12 grams of Carbon 12.
Calculate the number of water molecules in 05 mol of water. Of molecules in one gram of oxygen molecule. Web One mole 1 mol of a substance is the Avogadro constant number 602 10 23 of particles atoms molecules.
Web The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0012 kilogram of carbon 12. This relation is then used to convert a number of H 2 O molecules to grams. Web Before Using the Calculator.
Web Avogadros Number 6022x10 23. Simple calculator to estimate Avagadros constant of a substance. It expresses the number of elementary entities per mole of substance and it has the value.
Also one mole of nitrogen atoms contain s 602214179 1023 nitrogen atoms. Web Avogadros number is defined as the number of elementary particles molecules atoms compounds etc per mole of a substance. Web If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadros number of 602214154 x 10 23 particles per.
Web Avogadros number value. To input a scientific constant into a calculation press and then input the two-digit number that corresponds to the constant you want. Avogadros number is nothing but the amount of particles found in 1.
Web The Mole is an amount unit similar to pair dozen etc. Mole is a unit used to count very small things such as atoms or.

Esas Chemistry Rojas Electrical Engineering Review Center 2 Floor Yap Bullding 35 M Velez St Studocu

1 Using Avogadro S Number Calculate The Number Of Atoms In 0 005 Kilograms Of Carbon 2 If There Are X Atoms In 5 Grams Of Carbon How Many Atoms Are There In 5
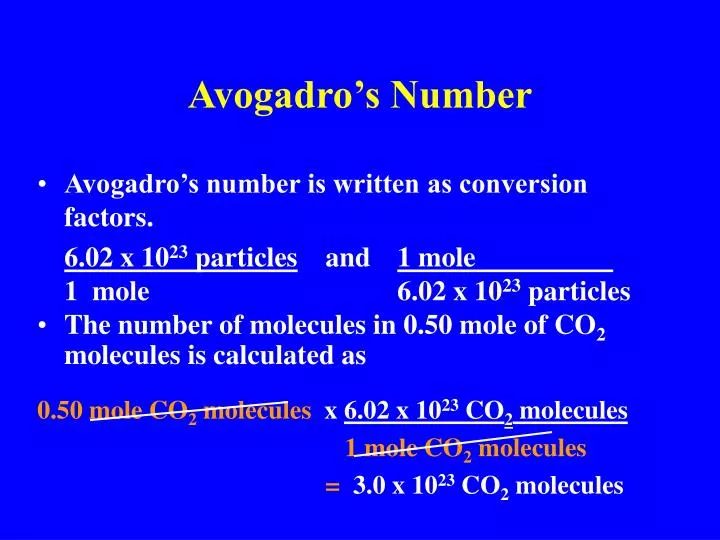
Ppt Avogadro S Number Powerpoint Presentation Free Download Id 4492286

Rbse Class 11 Chemistry Important Questions Chapter 5 States Of Matter
Conversion Factors And Avogadro S Number Youtube
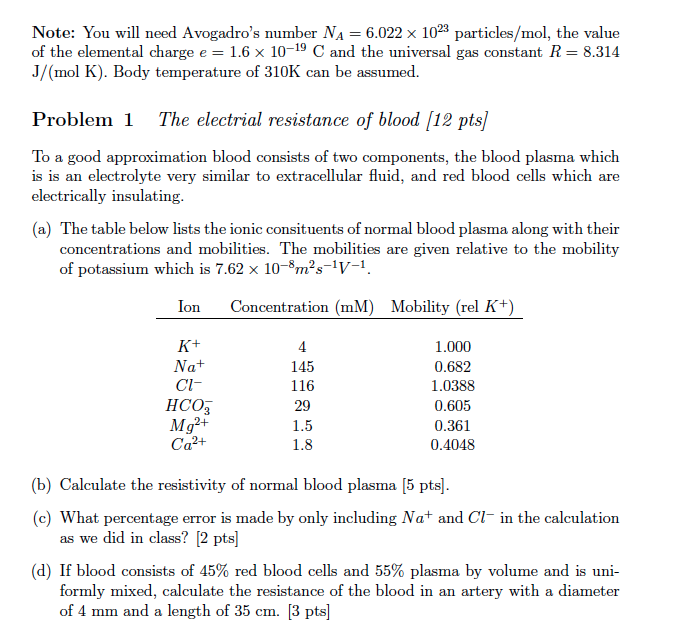
You Will Need Avogadro S Number Na 6 022 X 1023 Chegg Com
:max_bytes(150000):strip_icc()/Avogadro-58f7d6f35f9b581d5983024e.jpg)
Avogadro S Number To Calculate Mass Of A Single Atom

Gaseous State Docx

Toppr Ask Question

Calculating Moles Using Avogadro S Number Youtube
Full Article Hydrogenic Systems Frequency Standards And Fundamental Constants
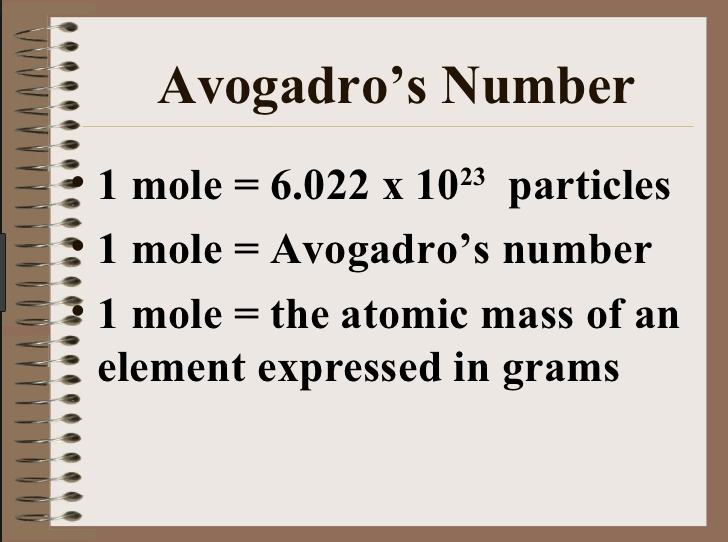
Avogadro S Number Chemistry Quiz Quizizz

What Is Avogadro S Number Howstuffworks
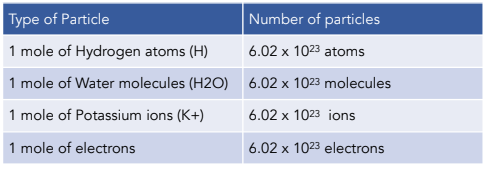
Amount Of Substance The Mole And The Avogadro Constant A Level Chemistry Study Mind

How To Calculate Molar Mass 7 Steps With Pictures Wikihow

1 Using Avogadro S Number Calculate The Number Of Atoms In 0 005 Kilograms Of Carbon 2 If There Are X Atoms In 5 Grams Of Carbon How Many Atoms Are There In 5
:max_bytes(150000):strip_icc()/the-periodic-table-of-the-elements-in-a-school-in-san-francisco--california--75471038-5a77c1650e23d90036d6c27c.jpg)
Example Of Molar Mass Calculation